Photoinitiators are essential components in ultraviolet (UV) curing systems. They play a crucial role in initiating the polymerization process, converting liquid materials into solid cured products.
In this article we will discuss about following topics:
1> What is a Photoinitiator for UV Curing?
2> Classifications of Photoinitiators
3> How does Photoinitiator Works?
4> Applicaitons of Photoinitiators in UV Curings.
1> What is a Photoinitiator for UV Curing?
In ultraviolet (UV) curing, the photoinitiator is a key substance. Photoinitiators are s special type of chemical compounds When exposed to ultraviolet light, the photoinitiator absorbs the energy of ultraviolet light of a specific wavelength and undergoes a chemical reaction, decomposing to generate active substances such as free radicals or cations. These active substances have very high reactivity and can trigger the polymerization and cross-linking reaction of monomers and oligomers in the system, thus rapidly transforming the liquid material into a solid state.
2> Classifications of Photoinitiators
Photoinitiators are mainly classified as follows:
I. Free radical photoinitiators
1. Cleavage type photoinitiators:
- After absorbing ultraviolet light, these photoinitiators undergo homolytic cleavage of molecules to generate two free radicals. Common ones are benzoin and its derivatives, such as benzoin dimethyl ether (651). Its chemical name is benzoin dimethyl ether (BDK). It appears as white crystals and is a highly efficient surface-drying photoinitiator. Its absorption wavelengths are 250nm and 340nm. It can be used to initiate free radical photopolymerization reactions and is commonly used in photocurable systems such as UV adhesives, UV coatings, and UV inks. It also has an inhibitory effect on styrene polymerization and can be used as a fragrance fixative.
- Characteristics: High initiation efficiency, large activity of generated free radicals, and can quickly initiate polymerization reactions. However, some cleavage type photoinitiators are easily oxidized in the air and have relatively poor stability.
2. Hydrogen abstraction type photoinitiators:
- After absorbing light energy, hydrogen abstraction type photoinitiators do not directly generate free radicals. Instead, they abstract hydrogen atoms from hydrogen donors in the system (such as compounds containing active hydrogen), thereby generating free radicals to initiate polymerization reactions. Common ones are benzophenone and its derivatives. Benzophenone photoinitiators usually appear as white to light yellow crystalline powders and are widely used in fields such as UV coatings and inks.
- Advantages: Good compatibility with the system and can absorb light energy in a relatively wide wavelength range. But the hydrogen abstraction reaction is relatively slow, which may lead to a slightly slower curing speed.
II. Cationic photoinitiators
Cationic photoinitiators generate cations after absorbing ultraviolet light and initiate cationic polymerization reactions. Common ones are diaryliodonium salts, triarylsulfonium salts, etc. For example, 784 (bis(2,6-difluoro-3-(pyrrol-1-yl)phenyl)titanocene) is a cationic photoinitiator. It appears as orange powder and is a highly efficient and active cationic ultraviolet photoinitiator that absorbs visible light (520nm). Its photoinitiation process is not free radical type. Instead, after absorbing light energy, it photoisomerizes into a cyclopentyl photoreactive intermediate and undergoes ligand substitution with the ester carbonyl group of acrylate in oligomers and reactive diluents to generate free radicals and initiate polymerization crosslinking.
- Characteristics of cationic photoinitiators: Not affected by oxygen in the air. The cured coating has a small shrinkage rate and strong adhesion. But the initiation speed is relatively slow and the price is relatively high.
III. Other types
1. Macromolecular photoinitiators:
- Usually, small molecule photoinitiators are connected to polymer chains through chemical bonds to form macromolecular photoinitiators with a certain molecular weight. This kind of photoinitiator has good solubility and stability and is not easy to migrate, which can effectively reduce the impact on the performance of cured products. For example, some macromolecular photoinitiators used in high-end UV coatings and inks can improve the weather resistance and durability of products.
- Disadvantage: The synthesis is relatively complex and the cost is relatively high.
2. Waterborne photoinitiators:
- Photoinitiators specially designed for waterborne UV curing systems. Since the waterborne system is different from the traditional organic solvent system, photoinitiators need to have good water solubility and stability in water. Common waterborne photoinitiators include sulfonates, phosphates, etc. This kind of photoinitiator is widely used in fields such as waterborne UV coatings and inks. It can meet environmental protection requirements and reduce the use of organic solvents.
- But the activity of waterborne photoinitiators may be relatively low, and a relatively high addition amount is required to achieve a better curing effect.
3> How Does Photoinitiator Works?
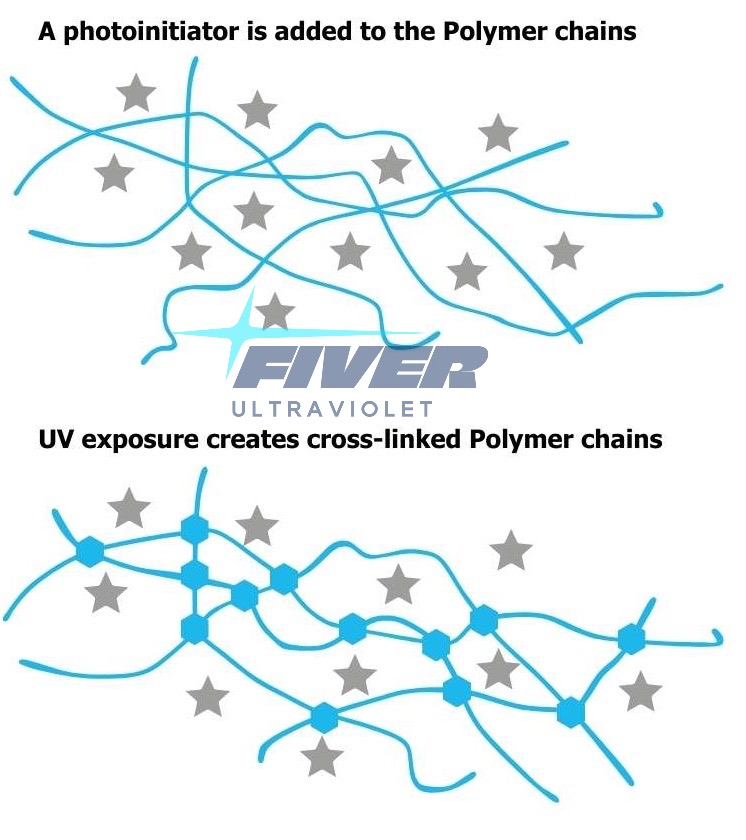
The working principle of photoinitiators in the ultraviolet curing process is as follows:
I. Absorbing ultraviolet light
Photoinitiators have a specific molecular structure and can absorb ultraviolet light of specific wavelengths. Different types of photoinitiators have different absorption wavelength ranges for ultraviolet light. For example, the absorption wavelengths of the common photoinitiator 1173 (2-hydroxy-2-methyl-1-phenyl-1-propanone) are 244nm, 278nm, and 322nm; the absorption wavelengths of 184 (1-hydroxycyclohexyl phenyl ketone) are 246nm, 280nm, and 333nm. When ultraviolet light irradiates a system containing a photoinitiator, the chemical bonds in the photoinitiator molecule absorb the energy of ultraviolet light, causing the molecule to transition from the ground state to the excited state.
II. Generating active species
The photoinitiator molecule in the excited state is unstable and will generate active species through different pathways. There are mainly the following two methods:
1. Free radical photoinitiators:
- Such photoinitiators will break chemical bonds and generate free radicals in the excited state. For example, after absorbing ultraviolet light, free radical photoinitiators such as 1173 and 184 will break carbon-hydrogen bonds or carbon-carbon bonds in the molecule to generate two free radicals. These free radicals have high reactivity and can quickly initiate free radical polymerization reactions of monomers or oligomers containing unsaturated double bonds.
2. Cationic photoinitiators:
- Cationic photoinitiators will generate cations in the excited state. For example, after absorbing visible light, 784 (bis(2,6-difluoro-3-(pyrrol-1-yl)phenyl)titanocene) photoisomerizes into a cyclopentyl photoreactive intermediate, and then undergoes ligand substitution with the ester carbonyl group of acrylate in oligomers and reactive diluents to generate cations. Cations can initiate ring-opening polymerization reactions of epoxy compounds and the like.
III. Initiating polymerization reactions
The generated active species (free radicals or cations) will quickly react with monomers or oligomers in the system to initiate polymerization reactions.
1. Free radical polymerization:
- Free radicals undergo addition reactions with unsaturated double bonds in monomers or oligomers to form new free radicals. This new free radical can continue to react with other monomers or oligomers, continuously repeating this process to continuously grow the molecular chain and finally form a cross-linked polymer network. For example, in UV coatings, the free radicals generated by free radical initiators will initiate the polymerization reaction of monomers such as acrylates, causing the coating to cure into a hard coating in a short time.
2. Cationic polymerization:
- Cations undergo ring-opening reactions with cyclic structures (such as epoxy compounds) in monomers or oligomers to form new cations. This new cation can continue to initiate the ring-opening polymerization reaction of other monomers to continuously grow the molecular chain. The cationic polymerization reaction speed is relatively slow, but the cured coating has advantages such as small shrinkage and strong adhesion. For example, in some high-performance UV adhesives, cationic initiators can initiate the polymeriz...
4> Applicaitons of Photoinitiators in UV Curings.
I. Coatings and inks
1. UV coatings:
- Characteristics and applications: UV coatings have advantages such as fast curing speed, high hardness, good abrasion resistance, and strong chemical corrosion resistance. They are widely used in surface coatings for furniture, floors, metal products, etc., to improve their appearance quality and protective performance. For example, after applying UV coatings on the surface of wooden furniture, a hard and smooth coating can be formed, which is not only beautiful but also can prevent scratches and abrasion.
- Role of photoinitiators: Under ultraviolet irradiation, photoinitiators decompose to generate free radicals or cations, which initiate the polymerization reaction of monomers and oligomers in the coating, causing the coating to quickly cure and form a film. Different types of UV coatings may require different types of photoinitiators to meet specific curing requirements and performance needs.
2. UV inks:
- Characteristics and applications: UV inks are mainly used in the uv printing industry and have characteristics such as fast drying speed, bright colors, strong adhesion, and good light resistance. They are suitable for various printing materials such as paper, plastic, and metal. For example, in packaging printing, UV inks can cure quickly, improving production efficiency while ensuring the quality and durability of printed products.
- Role of photoinitiators: Under the action of ultraviolet light, photoinitiators initiate the polymerization reaction of resins and monomers in the ink, causing the ink to dry quickly and firmly adhere to the printing material. The selection of photoinitiators is crucial for aspects such as the curing speed, color stability, and printability of UV inks.
II. Adhesives
UV adhesives:
- Characteristics and applications: UV adhesives are adhesives that cure rapidly under ultraviolet irradiation. They have advantages such as high bonding strength, good transparency, and good weather resistance. They are widely used in fields such as electronics, optics, and healthcare, such as mobile phone screen assembly, optical lens bonding, and medical device assembly. For example, in mobile phone manufacturing, UV adhesives can quickly bond the phone screen and body, improving production efficiency and product quality.
- Role of photoinitiators: Under the excitation of ultraviolet light, photoinitiators generate free radicals or cations, which initiate the polymerization reaction of monomers and oligomers in the adhesive, causing the adhesive to quickly cure and achieve a firm bond to the bonded materials. Different UV adhesives may require different types of photoinitiators to adapt to different bonding materials and application environments.
III. 3D printing materials
Photosensitive resins:
- Characteristics and applications: Photosensitive resins are materials used for 3D printing and have characteristics such as high precision, high resolution, and smooth surface. Under ultraviolet irradiation, photosensitive resins can cure quickly to form complex three-dimensional structures. They are widely used in fields such as model making, jewelry design, and dental medicine. For example, in the dental field, photosensitive resins can be used to make personalized dental crowns, bridges, and other restorations through 3D printing technology.
- Role of photoinitiators: Photoinitiators are an indispensable component in photosensitive resins. Under the action of ultraviolet light, they initiate the polymerization reaction of the resin, causing the liquid photosensitive resin to quickly cure into a solid state. The type and dosage of photoinitiators will affect aspects such as the curing speed, mechanical properties, and precision of photosensitive resins.
IV. Electronic materials
1. Photoresists:
- Characteristics and applications: Photoresists are important materials for semiconductor manufacturing and microelectronic processing. They have characteristics such as high resolution, high sensitivity, and good chemical stability. Under ultraviolet exposure, photoresists undergo chemical reactions to form specific patterns and are used to manufacture electronic devices such as integrated circuits and flat panel displays. For example, in integrated circuit manufacturing, photoresists can form tiny circuit patterns through processes such as ultraviolet exposure and development.
- Role of photoinitiators: Photoinitiators play a key role in photoresists. Under ultraviolet irradiation, they initiate the chemical reaction of the photoresist, causing a change in the solubility of the photoresist, thereby realizing pattern transfer and etching. Different types of photoresists require different types of photoinitiators to meet different process requirements and performance needs.
V. Other materials
1. Composite materials:
- Characteristics and applications: Ultraviolet-cured composite materials usually consist of fiber-reinforced materials (such as glass fiber, carbon fiber, etc.) and resin matrices. These composite materials have advantages such as high strength, high stiffness, and lightweight, and are widely used in fields such as aerospace, automobiles, and sporting goods. For example, in the aerospace field, ultraviolet-cured composite materials can be used to manufacture aircraft parts and satellite structural components.
- Role of photoinitiators: Under the action of ultraviolet light, photoinitiators initiate the polymerization reaction of the resin matrix, causing the composite material to quickly cure, improving production efficiency and product performance. The selection of photoinitiators needs to consider factors such as the composition of composite materials, processing technology, and performance requirements.
2. Dental materials:
- Characteristics and applications: In addition to the photosensitive resins for 3D printing mentioned above, the dental field also uses other ultraviolet-cured materials, such as dental filling materials and dental adhesives. These materials have good biocompatibility, abrasion resistance, and aesthetics, and are suitable for dental restoration and cosmetology. For example, in dental filling treatment, ultraviolet-cured filling materials can cure quickly and tightly bond to teeth, restoring the shape and function of teeth.
- Role of photoinitiators: Under ultraviolet irradiation, photoinitiators initiate the polymerization reaction of dental materials, causing the materials to quickly cure and improve treatment efficiency and patient comfort. The safety and stability of photoinitiators are crucial for the clinical application of dental materials.
Post time:2024-10-07
