UV Curing Technology and Their Applications
Curing is the term used to describe the chemical reactions that take place during polymerization processes. Polymerization involves the bonding or crosslinking of separate molecules and shorter polymer chains to create longer, stronger, and more rigid polymer chains. Although various substances have polymeric structures, the fields of polymer chemistry and polymer engineering are mainly concerned with materials and processes related to plastics and rubbers.
UV photopolymerization, also known as radiation curing or UV curing, is a specific type of polymerization where ultraviolet energy induces crosslinking in UV formulated materials like inks, coatings, adhesives, and extrusions. This technology has been successfully used for almost seventy-five years in various manufacturing processes across different industries. UV curing offers desirable material properties and quick, space-efficient installations that rapidly transform wet materials into solid structures.
In many cases, UV formulations consist of all solids or high solids materials without liquid carriers. The solid particles are small enough and abundant enough to be applied like liquids on a variety of surfaces. Although some may refer to the rapid change in structure during UV curing as drying, UV formulated inks, coatings, and adhesives undergo curing rather than drying.
In contrast, conventional water and solvent-borne formulations contain liquid carriers that help in applying the desired solids content onto surfaces using various methods like printing, spraying, coating, and dispensing. Once applied, the carrier evaporates or dries using forced air or thermal dryers, leaving behind only the solid particles on the surface. In the case of solvent carriers, regulations often require the evaporated solvent vapors to be burned in afterburners before being released into the environment.
For processes that do not use UV light for curing, long drying tunnels and energy-consuming ovens are typically needed to drive evaporation. Unlike UV curing, drying processes do not bring about molecular changes in the chemistry; they simply leave behind residual solids on the surface of materials like plastic and metal or slightly absorbed into porous structures like wood and paper. These residual solids are prone to scratching, marring, scuffing, chemical damage, and weathering. In many cases, clear top coatings and other protective measures such as film lamination are necessary when using water and solvent-borne materials.
UV curing results in long polymer chains and strong molecular structures that provide desirable physical, mechanical, and aesthetic properties, enhancing the appearance, quality, and performance of various everyday items. Manufacturers use UV curing to create vibrant ink colors, special visual and tactile effects, strong bonds, superior chemical resistance, weathering resistance, and desirable surface qualities like hardness, slip resistance, and impact resistance. UV curing is environmentally friendly as it eliminates energy-consuming ovens and drying tunnels, volatile organic compounds (VOCs), and hazardous air pollutants (HAPs).
Due to the rapid chemical crosslinking process of UV curing, post-cure processing, converting, packing, assembling, filling, and shipping can all be done immediately after the final UV curing station.
Electromagnetic spectrum
The electromagnetic spectrum encompasses the entire range of light in the universe, including light emitted from objects on Earth and celestial bodies. Light, which always radiates outward from its source, is commonly known as electromagnetic radiation or radiation. The electromagnetic spectrum is visually represented in the illustration below.
![]()
The electromagnetic spectrum is divided into various segments, including gamma rays, x-rays, ultraviolet, visible light, infrared, and radio waves. Within the ultraviolet bandwidth, there are further subdivisions such as vacuum UV, UVC, UVB, UVA, and UVV. Radio waves are categorized into radar, TV, FM, and AM, with microwaves often considered a distinct band between infrared and radio waves. Each segment of the spectrum is defined by its wavelength, frequency, and photonic energy, with visible light being the only segment that produces color when interacting with objects.
The boundaries between these segments are not fixed, and there are no universally established wavelengths that mark the transition from one segment to another. Different industries, organizations, standards, and measurement tools may reference slightly different ranges within the spectrum. For UV curing purposes, specific ranges are typically utilized.
The vacuum UV segment of the electromagnetic spectrum ranges from 100 to 200 nm and is named as such because these wavelengths travel effectively in a vacuum but do not pass through air. Vacuum UV emitted from curing systems is most effective in photopolymerization processes when operated in nitrogen inerted environments to prevent interference. Depending on the oxygen levels in the inerted environment, vacuum UV may generate ozone.
UVC, ranging from 200 to 280 nm, is often referred to as short UV or germicidal UV and is not visible to the human eye. Ozone production typically occurs at UVC wavelengths of 240 nm and below. UVC wavelengths are absorbed at the surface of UV chemistry and facilitate surface curing.
UVB spans from 280 to 315 nm and is also invisible to the human eye. UVB wavelengths penetrate deeper into the chemistry compared to UVC but not as deeply as UVA and UVV.
UVA, ranging from 315 to 400 nm, represents the largest portion of UV energy and is known as long UV. UVA is at the lower limit of what the human eye perceives as color and can penetrate deeply into UV chemistry.
UVV, ranging from 400 to 450 nm, is a segment of the electromagnetic spectrum perceived as color by the human eye, hence the V for visible. These wavelengths overlap slightly with the visible spectrum and penetrate deeply into UV chemistry.
The ultraviolet segment spans from 100 to 450 nm, with the boundary between UV and visible wavelengths not precisely defined but generally considered to be between 400 and 450 nm. UV wavelengths are just beyond what the human eye perceives as the color violet.
Visible light ranges from 400 to 700 nm and encompasses all colors, with different wavelengths or combinations of wavelengths creating various hues when reflected off objects. The visible spectrum is often depicted as a rainbow.
Infrared wavelengths, from 700 nm to 0.1 mm, are beyond what the human eye sees as the color red. Some infrared wavelengths emit heat, while others do not. Infrared wavelengths play a significant role in the heat radiated from electrode and microwave UV curing systems, although quasi-monochromatic UV LED curing systems do not emit infrared.
Microwaves, spanning from 0.1 mm to 10 cm, are a narrow band at the edge of radar and the broader radio wave segment of the electromagnetic spectrum. Microwaves can be utilized to power a specific type of UV curing lamp in electrodeless or microwave curing systems.
Conventional broadband UV sources like microwave and electrode arc lamps emit a wide range of UV wavelengths, including UVA, UVB, UVC, and UVV, along with visible and infrared light. On the other hand, commercially available LED curing sources emit narrower bands of UVA closer to the visible spectrum, typically at 365, 385, 395, and 405 nm. This difference in wavelength output means that conventional broad-spectrum UV chemistry may not cure effectively with UV LEDs and requires reformulation to work with longer UVA and UVV wavelengths.
Fortunately, companies in the ink, coating, and adhesive industries are adapting by designing UV LED chemistry that can be cured using both LED and conventional lamps. This dual-cure capability is becoming more common, allowing for a smoother transition towards UV LED curing. In the coming years, we can expect to see a shift towards formulations that can be cured using both types of lamps, driving the industry towards greater adoption of UV LED technology.
Wavelength, Frequency, and Photonic Energy
In the electromagnetic spectrum, each segment is defined by its wavelength, frequency, and photonic energy. Wavelength is the distance between corresponding points on a wave of light and is symbolized by the Greek letter lambda (λ). Frequency, on the other hand, is the rate at which a specific wavelength occurs, measured in cycles per second or Hertz (Hz), and typically denoted by the letters (f) or (ν). The relationship between frequency and wavelength is determined by the speed of light, where frequency equals the speed of light divided by the wavelength. As wavelengths become longer, frequency decreases, while shorter wavelengths correspond to higher frequencies.
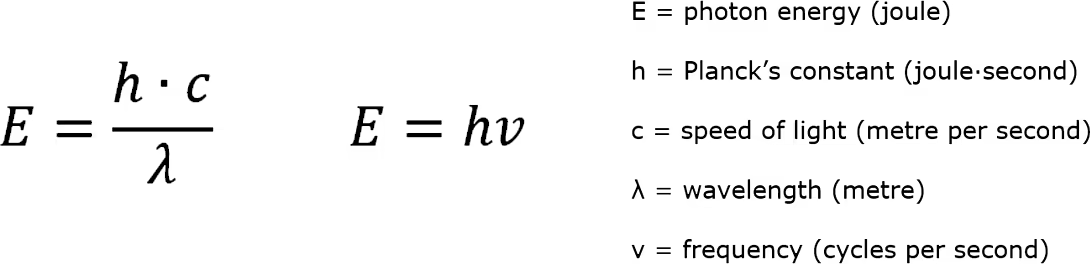
The Planck-Einstein Relationship states that shorter wavelengths, like UVC, carry more energy compared to longer wavelengths such as UVA. This means that ultraviolet wavelengths, in general, have significantly more energy than visible light, infrared, microwaves, and radio waves, but less energy than x-rays and gamma rays. This relationship helps to explain the varying energy levels across different segments of the electromagnetic spectrum.
Irradiance (W/cm2)
Irradiance refers to the radiant power that reaches a surface per unit area and is typically measured in watts or milliwatts per square centimetre (W/cm2 or mW/cm2). It is also known as power density or watt density. While technically incorrect, irradiance is often referred to as intensity, with the understanding that it means the same thing. When measuring irradiance, it is important to consider the peak values across a range of ultraviolet wavelengths or within specific segments like UVC, UVB, UVA, and UVV.
Irradiance is a fixed value for a given distance between a curing surface and a UV curing system, regardless of production line speed or exposure time. This means that the irradiance at the curing surface will increase with higher lamp power settings and decrease with lower settings. The value of irradiance remains constant at the curing surface as long as the lamp power setting and distance are unchanged, regardless of the production line speed. It is essential to reference the radiometer used for measurement and the location of the radiometer concerning the UV source when reporting irradiance values.
A dynamic irradiance profile can take on various shapes, but it typically resembles a bell curve as illustrated graphically. Each point on the irradiance profile represents the peak irradiance at a specific moment in time, with the peak of the bell curve indicating when the cure surface passes through the focal area of a mercury vapor lamp or the center of a UV LED lamp. Sources with higher peak irradiance will have a taller peak in the profile, while sources with lower peak irradiance will have a shorter peak.
The width of the profile is influenced by the width of the UV lamphead, as well as the speed of the process line or exposure time. This dynamic nature of the irradiance profile is important to consider when optimizing UV curing processes and ensuring uniform curing across the surface being treated.
Energy density refers to the total radiant energy that reaches a surface per unit area and is measured in J/cm2 or mJ/cm2. It is calculated by integrating irradiance (W/cm2 or mW/cm2) over the exposure time, which is represented by the area under the irradiance profile. By slowing the line speed, increasing dwell time, adding more curing sources, or using wider heads for UV LEDs, the exposure time and energy density can be increased.
In a UV curing application, once the correct spectral output and minimum threshold irradiance are achieved at the cure surface, the energy density becomes the most critical factor influencing the rate and extent of curing. While irradiance represents the rate of energy transfer, energy density reflects the total energy delivered. UV curing systems that offer higher energy density allow for faster curing at higher line speeds compared to systems with lower energy densities.
Although commonly referred to as dose, energy density is the technically correct term. Dose is often used interchangeably with energy density, with the understanding that it refers to absorbed energy. Absorbed energy is challenging to quantify accurately, whereas energy density can be estimated using UV radiometers and energy density test strips. When reporting energy density values obtained through radiometers, it is essential to specify the meter and line speed used for measurement.
UV curing relies on chemical reactions that are initiated by photoinitiators present in specially formulated inks, coatings, adhesives, and extrusions. These photoinitiators, when exposed to UV light in the range of 100 to 450 nm, absorb energy and produce free radical or cationic species. These excited species drive reactions through initiation, propagation, and termination, leading to rapid changes in the molecular structure of the raw material components. In UV curing applications, free radical curing mechanisms are predominantly used, with cationic applications making up a small percentage of UV curable chemistry.
Free radical curing involves the generation of free radicals that facilitate crosslinking reactions. Continuous and direct UV exposure is necessary to initiate and propagate the free radical cure. The reactions terminate quickly, resulting in the formation of new materials with desired properties. However, free radical chemistry can be affected by oxygen, especially at the cure surface. Oxygen molecules can interfere with the curing process by depleting free radicals or weakening their strength. To counteract oxygen inhibition, methods such as increasing lamp irradiance, curing in a nitrogen environment, and adjusting the chemistry are commonly employed.
Another less commonly used curing mechanism is cationic curing, which involves the generation of cations that drive crosslinking reactions. Unlike free radical curing, cationic curing does not require continuous or total direct line-of-sight UV exposure for propagation. Depending on the specific chemistry and application, cationic curing processes can propagate over minutes, hours, or even days before reaching full termination. Cationic chemistry is sensitive to moisture and temperature, both of which can impact crosslinking. Therefore, it is important to monitor and control ambient environmental conditions when working with cationic chemistry.
UV curable formulations, whether based on free radical or cationic mechanisms, are composed of commercially available materials. These raw materials are typically categorized into different types:
- Photoinitiators: These molecules absorb UV energy within a specific range of wavelengths and drive photopolymeric reactions by creating free radicals or cations. Photoinitiators typically make up 0.5 to 15% of the total UV formulation by weight.
- Monomers: These are resin molecules with relatively low molecular weight and simple structure that can bond with themselves or other similar molecules to form UV crosslinked polymers. Monomers are used as reactive diluents to adjust viscosity and influence cured material properties. They can constitute up to 50% of the total formulation weight and, in some cases, may be partially or entirely replaced by water or solvent.
- Oligomers: These resin molecules, also with low molecular weight and simple structure, play a key role in forming the backbone of crosslinked materials and influencing various properties of the cured material. Oligomers typically make up 50 to 80% of the formulation by weight.
- Additives: These components are added to adjust the properties of the mixture before curing and the properties of the polymer after curing. Additives include flow modifiers, defoamers, dispersants, and light stabilizers, among others. Additives, along with pigments, constitute the smallest portion of the overall UV mixture.
- Pigments: These additives provide color to the formulations.
Chemists blend photoinitiators, monomers, oligomers, additives, and sometimes pigments to create UV curable inks, coatings, adhesives, and extrusions. When exposed to the optimal UV wavelength and irradiance combination, free radical or cationic species are generated within the chemistry, leading to rapid photopolymerization in fractions of a second. The speed and effectiveness of crosslinking depend on the raw material blend, the UV source's wavelengths, irradiance, and energy density, the application, the production line setup, and the choice of free radical or cationic curing mechanisms.
The liquid-like consistency of UV formulations allows for versatile application methods such as extrusion, spraying, jetting, dispensing, rolling, dipping, printing, sputtering, vacuuming, or flooding onto various surfaces and materials. Once applied, exposure to ultraviolet energy triggers a propagating and crosslinking reaction that efficiently bonds the molecules into solid, homogeneous structures.
The process of UV curing involves a series of interconnected manufacturing steps that occur before and after the crosslinking reactions take place. It is essential to identify, consider, evaluate the impact of, and maintain all these steps to consistently produce products that meet quality and performance requirements.
Some of the process steps related to UV curing include:
1. Evaluation of the cure surface: Assessing the shape, structure, material type, quality, surface energy, and cleanliness of the cure surface to determine the need for pretreatment, the appropriate formulation, material handling, and UV lamphead orientation.
2. Pretreatment and substrate modification: Cleaning, static removal, or surface energy enhancement of part and material surfaces to facilitate wetting of inks, coatings, and adhesives, and improve adhesion.
3. Material handling: Ensuring that material surfaces are fed through production equipment in sheets, webs, or three-dimensional parts to enable even application of the formulation and exposure to UV light at a defined offset distance from the lamphead.
4. Formulation delivery equipment: Applying inks, coatings, and adhesives using methods such as spraying, jetting, dispensing, rolling, dipping, or flooding, matching the equipment to the formulation and part characteristics.
5. Evaporation of liquid carriers: Some UV formulations contain water or solvent carriers that need to be evaporated before UV exposure, either through natural evaporation during part travel or with a drying mechanism.
6. UV cure: Ensuring direct line-of-sight UV exposure, matching UV wavelengths to photoinitiators, achieving the required irradiance and energy density for curing at the desired line speed.
7. Ancillary process equipment: Using additional equipment like nitrogen inertion systems, chilled rollers, pressurized lampheads, clean rooms, UV filters, and UV measurement systems to assist with curing or manage process conditions.
8. Post-cure processing: Converting, finishing, assembling, filling, packing, and shipping the cured parts or material surfaces.
9. Quality inspections: Establishing quantifiable targets for quality and performance specifications, monitoring process variables during production, and making corrections as needed.
10. End-use performance: Considering the final product's conditions of use to determine the preceding process steps and ensure they meet the end-use requirements.
11. Qualifications, approvals, and certifications: Obtaining necessary qualifications, approvals, and certifications from purchasing parties to ensure the overall production process meets their specific requirements and objectives.
While the provided bullet list offers a comprehensive overview of a UV curing process, it may not cover all potential activities. Each UV curing application and manufacturing line will have unique characteristics that could introduce additional process steps. It is essential to evaluate UV curing applications, the manufacturing line, post-cure processing, and final product use as a complete process, considering the potential impact of each step on the others. Once established, a UV curing process is reliable, repeatable, and controllable.
UV curing allows manufacturers to create products with desirable physical, mechanical, and aesthetic properties, enhancing the appearance, quality, and performance of various everyday items. Understanding UV curing basics, light science, UV chemistry, and process variables helps in selecting the right UV curing source and formulation, integrating them properly, controlling the process, and consistently producing high-quality products.
Post time:2024-06-27

